About the study
IMPRESS-Norway is a prospective, non-randomized clinical trial evaluating efficacy of commercially available, anti-cancer drugs prescribed for patients with advanced cancer diagnosed with potentially actionable alterations as revealed by molecular diagnostics. IMPRESS-Norway is a nation-wide study and all hospitals with an oncology and / or hematology department will be invited to participate in the study.
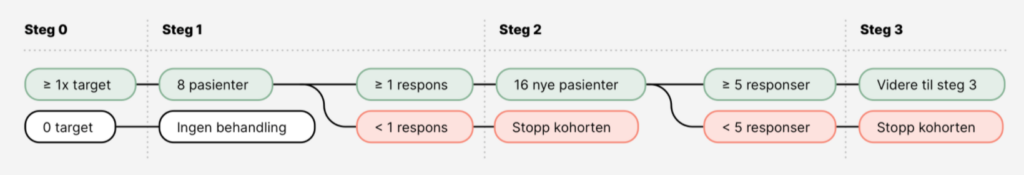
Patients that have an advanced malignancy already treated with standard treatment, acceptable performance status and organ function can be referred to molecular profiling to investigate the possibility of experimental treatment (inclusion in a clinical study such as IMPRESS-Norway). Eligible patients are first offered a gene panel analysis that maps 523 genes (TSO500) performed by Infrastructure for Precision Diagnostics (InPreD), which presents the results to the IMPRESS-team and the treating oncologist in a national molecular tumor board meeting. Specialists from various disciplines such as bioinformatics, molecular biology, oncology and pathology participate in the national molecular tumor board to discuss treatment options.
IMPRESS-Norway is a clinical study that investigates the effect of drugs that have already been approved for some tumor types, on new tumor types. Patients with molecular alterations that indicates effect of targeted treatment can be offered this through IMPRESS-Norway if the drug in question is part of the drug portfolio. Thus, the study tests approved drugs on new indications, so-called off-label treatment, based on the patient’s molecular profile rather than the location of the cancer.
The aim of IMPRESS-Norway is to offer targeted treatment to a higher number of Norwegian cancer patients (Helland Å et al. Improving public cancer care by implementing precision medicine in Norway: IMPRESS-Norway. J Transl Med. 2022 May 14;20(1):225. PMID: 35568909)